Our company covers an area of 900 square meters where various laboratories and warehouses are located for the study and the production (storage tower for electronic components, manufacturing robots, ESD protection, controlled temperature…).
Our stock is electronically managed by the ERP (Enterprise Resource Planning) system HELIOS II to ensure rapid re-supplies of components. The electronic components are stored at a storage area of 130 square meters ensuring ESD (Electrostatic discharge) and MSL (Moisture sensitivity level) requirements. A buffer stock is in place to ensure continuity of the production process within each EFS.
Quality standards at all levels to meet your deadlines
Thanks to our experience and quality at all levels, we are committed to delivering your products on time and providing you with the best customer service possible.
Partner for your process customization and automation
Our knowledge of blood transfusion and its requirements make ACÉMIS France, a preferred partner for the automation and modernization of your whole blood transformation processes.
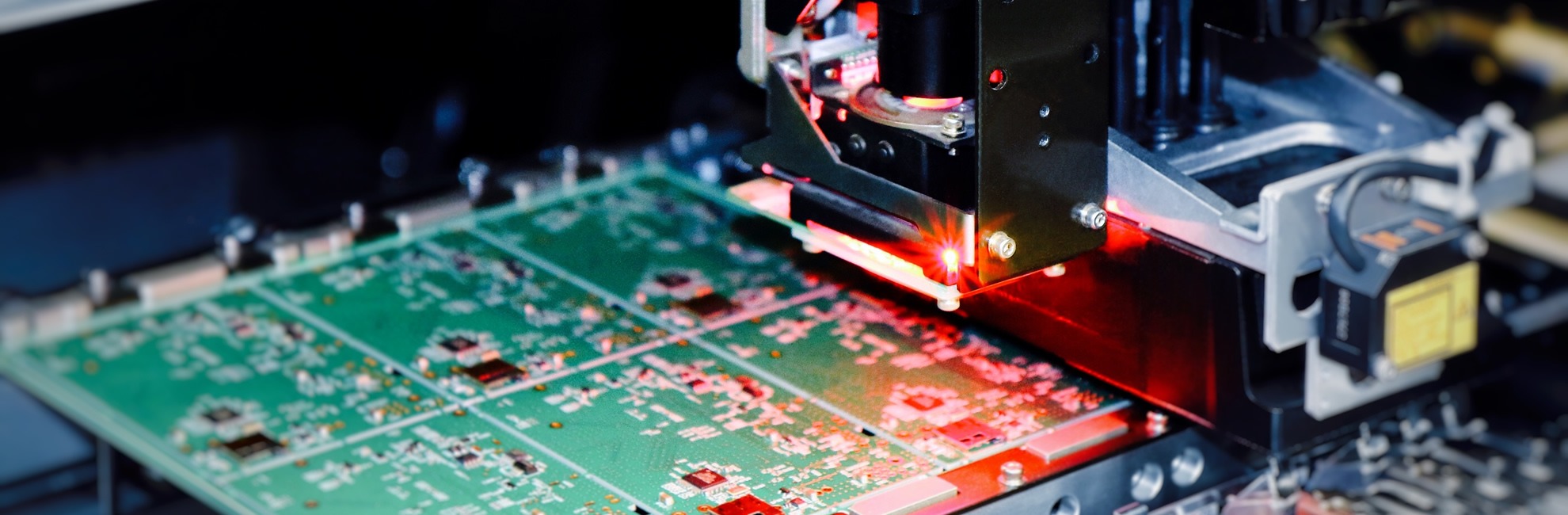
Electronic subcontracting
Our electronic subcontracting sector has accompanied industrials from the South-West of France in the assembly of electronic boards for nearly 30 years.
Since 1994, ACEMIS France has been designing and manufacturing medical equipment. More than 2,000 blood component separation machines worldwide.
© C-LAB, C-RAMP, C-CAD, and C-BAC are equipment manufactured by ACÉMIS France.
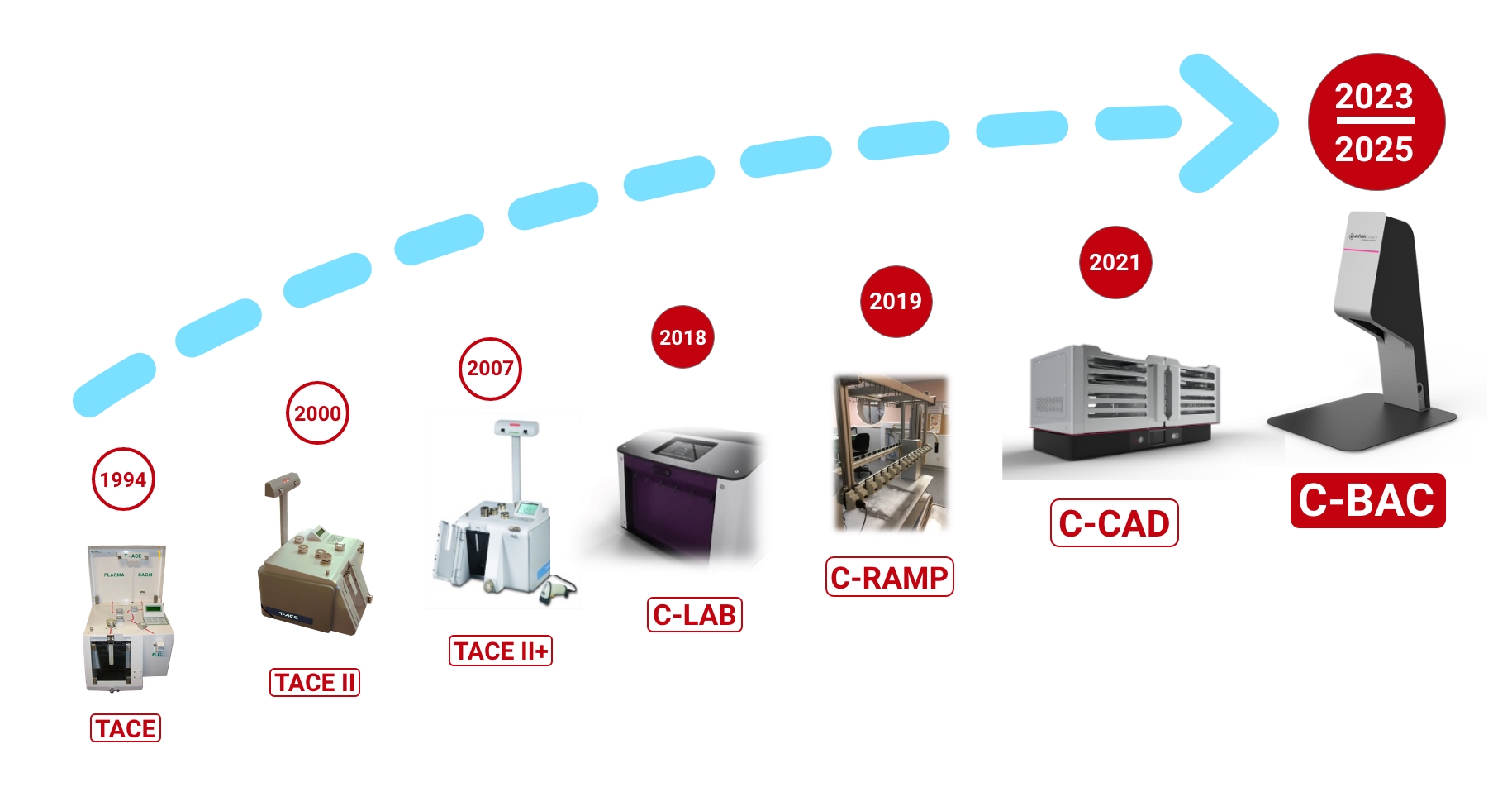
Company’s history
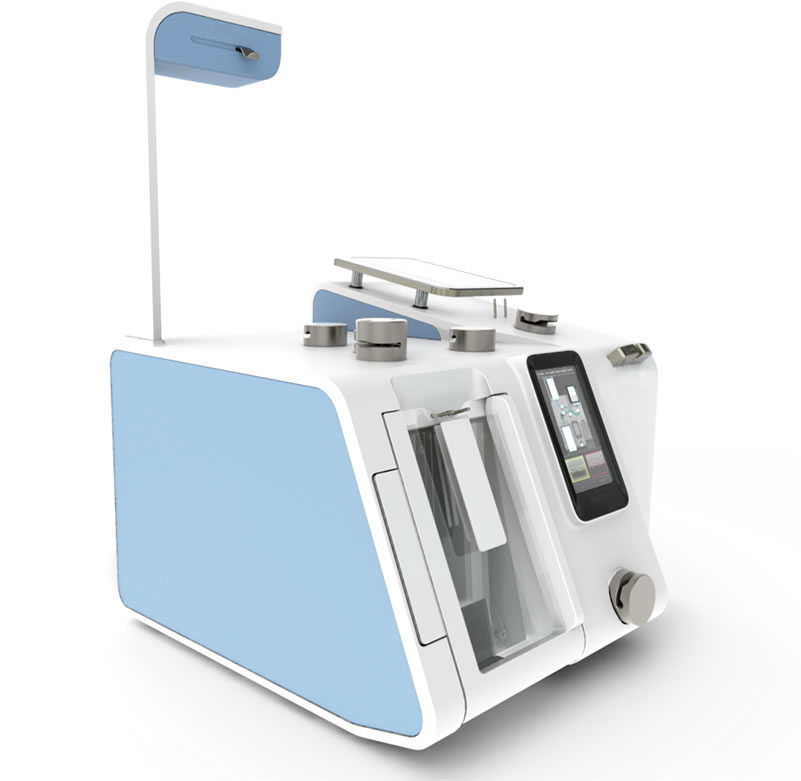
Success Story
T-ACE II+, more than 2000 devices around the world!
Developed and manufactured by ACÉMIS France, the T-ACE component extractor has progressed as a world reference since its launch in 1994.
Over the years, T-ACE gave way to T-ACE II and then T-ACE II+ with more than 2000 running devices around the world.
ACÉMIS France has the appropriate skills and know-how to improve the current generation of blood component extractors.